Products
CHLORFENAPYR
Insecticide, acaricide
pyrazole (acaricide) analogue
NOMENCLATURE
Common name chlorfenapyr (BSI, pa ISO, ANSI)
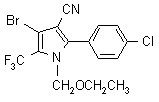
IUPAC name 4-bromo-2-(4-chlorophenyl)-1-ethoxymethyl-5-trifluoromethylpyrrole-3-carbonitrile
Chemical Abstracts name 4-bromo-2-(4-chlorophenyl)-1-(ethoxymethyl)-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile
CAS RN [122453-73-0]
PHYSICAL CHEMISTRY
Mol. wt. 407.6 M.f. C15H11BrClF3N2O Form White
solid. M.p. 100-101 ºC KOW logP
= 4.83 Solubility Practically insoluble in water.
Soluble in acetone, diethyl ether, dimethyl sulfoxide, tetrahydrofuran,
acetonitrile, and alcohols.
COMMERCIALISATION
History Under development by American Cyanamid Co.
(now BASF AG).
APPLICATIONS
Biochemistry Oxidative removal in vivo of
the N-ethoxymethyl group generates the active species, which
is a mitochondrial uncoupler. Mode of action Insecticide
and acaricide with mainly stomach and some contact action. Exhibits
good translaminar but limited systemic activity in plants. Uses Control
of many species of insects and mites, including those resistant to
carbamate, organophosphate and pyrethroid insecticides and also chitin-synthesis
inhibitors, in cotton, vegetables, citrus, top fruit, vines and soya
beans. Among pests resistant to conventional products which are controlled
by chlorfenapyr are Brevipalpus phoenicis (leprosis mite), Leptinotarsa
decemlineata (Colorado potato beetle), Helicoverpa spp., Heliothis spp., Plutella
xylostella (diamond-back moth) and Tetranychus spp. Its
use in resistance management programmes for control of various cotton
pests is under evaluation. Phytotoxicity No
phytotoxicity observed at field use rates. Formulation
types EC; SC. Selected tradenames: 'Pirate'
(cotton) (BASF); 'Stalker' (BASF)
