Products
OXYFLUORFEN
Herbicide: diphenyl ether
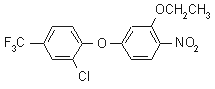
NOMENCLATURE
Common name oxyfluorfen (BSI, E-ISO, ANSI,
WSSA); oxyfluorfène ((m) F-ISO)
IUPAC name 2-chloro-a,a,a-trifluoro-p-tolyl 3-ethoxy-4-nitrophenyl
ether
Chemical Abstracts name 2-chloro-1-(3-ethoxy-4-nitrophenoxy)-4-(trifluoromethyl)benzene
CAS RN [42874-03-3] EEC no. 255-983-0 Development
codes RH-2915 (Rohm & Haas)
PHYSICAL CHEMISTRY
Mol. wt. 361.7 M.f. C15H11ClF3NO4 Form Orange,
crystalline solid. M.p. 85-90 ºC; (tech.,
65-84 ºC) B.p. 358.2 ºC (decomp.) V.p. (pure
a.i.) 0.0267 mPa (25 ºC) KOW logP = 4.47 S.g./density 1.35
(73 ºC) Solubility In water 0.116 mg/l
(25 ºC). Readily soluble in most organic solvents, e.g. acetone
72.5, cyclohexanone, isophorone 61.5, dimethylformamide >50, chloroform
50-55, mesityl oxide 40-50 (all in g/100 g, 25 ºC). Stability No
significant hydrolysis in 28 d at pH 5-9 (25 ºC). Decomposed rapidly
by u.v. irradiation; DT50 3 d (room temperature). Stable up to 50 ºC.
APPLICATIONS
Biochemistry Protoporphyrinogen oxidase inhibitor. Mode
of action Selective contact herbicide, absorbed more readily
by the foliage (and especially the shoots) than by the roots, with
very little translocation. Uses Control of annual
broad-leaved weeds and grasses in a variety of tropical and subtropical
crops, by pre- or post-emergence application at rates in the range
0.25-2.0 kg a.i./ha. Particular crops include tree fruit (including
citrus), vines, nuts, cereals, maize, soya beans, peanuts, rice, cotton,
bananas, peppermint, onions, garlic, ornamental trees and shrubs, and
conifer seedbeds. Phytotoxicity Soya beans and
cotton may be injured by contact with oxyfluorfen. Formulation
types EC; GR.
