Products
RIMSULFURON
Herbicide: sulfonylurea
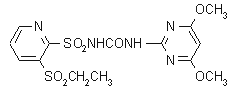
NOMENCLATURE
Common name rimsulfuron (BSI, pa E-ISO, ANSI)
IUPAC name 1-(4,6-dimethoxypyrimidin-2-yl)-3-(3-ethylsulfonyl-2-pyridylsulfonyl)urea
Chemical Abstracts name N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(ethylsulfonyl)-2-pyridinesulfonamide
CAS RN [122931-48-0] Development codes DPX-E9636
(Du Pont)
PHYSICAL CHEMISTRY
Composition Tech. is 99%. Mol. wt. 431.4 M.f. C14H17N5O7S2 Form Colourless
crystals. M.p. 176-178 ºC V.p.
1.5 × 10-3 mPa (25 ºC) KOW logP = 0.288 (pH
5), -1.47 (pH 7) (25 ºC) S.g./density 0.784
(25 ºC) Solubility In water (25 ºC) <10
mg/l (unbuffered); 7.3 g/l (buffered, pH 7). Stability On
hydrolysis (25 ºC), DT50 4.6 d (pH 5), 7.2 d (pH 7), 0.3 d (pH
9). pKa 4.0
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS
or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential
amino acids valine and isoleucine, hence stopping cell division and
plant growth. Crop selectivity derives from rapid, selective metabolism
by contraction of the sulfonylurea group and ring migration (L. Martinetti
et al., Proc. Br. Crop Prot. Conf. - Pests Dis., 1995, 1, 405)
and by hydroxylation on the pyrimidine ring, followed by glucose conjugation
(M. K. Koeppe, IUPAC 5E-003 (1998); see Environmental Fate. Mode
of action Selective systemic herbicide, absorbed by the foliage
and roots, with rapid translocation to the meristematic tissues. Uses Rimsulfuron
is a post-emergence sulfonylurea herbicide that effectively controls
most annual and perennial grasses and several broad-leaved weeds in
maize. Also used in tomatoes and potatoes. The target rate for most
situations is 15 g a.i./ha. Rimsulfuron has a wide crop-safety margin
under most conditions. Formulation types WG.
