Products
CYPROCONAZOLE
Fungicide
FRAC 3; DMI: triazole
NOMENCLATURE
Common name cyproconazole (BSI, draft E-ISO, (m)
draft F-ISO)
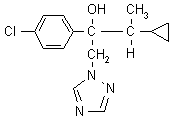
IUPAC name (2RS,3RS;2RS,3SR)-2-(4-chlorophenyl)-3-cyclopropyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
Chemical Abstracts name a-(4-chlorophenyl)-a-(1-cyclopropylethyl)-1H-1,2,4-triazol-1-ethanol
CAS RN [94361-06-5] for (2RS,3RS;2RS,3SR)
isomers; [113096-99-4] for unstated stereochemistry; [94361-07-6] for
(2RS,3SR) isomers Development codes SAN
619 F (Sandoz)
PHYSICAL CHEMISTRY
Composition It is a mixture (c. 1:1) of 2
diastereoisomers. Mol. wt. 291.8 M.f. C15H18ClN3O Form Colourless
solid. M.p. 106-109 ºC B.p. >250 ºC V.p.
3.46 ´ 10-2 mPa (20 ºC) KOW logP
= 2.91 (pH 7) S.g./density 1.259 g/cm3 Solubility In
water 140 mg/l (25 ºC). In acetone 230, ethanol 230, dimethyl
sulfoxide 180, xylene 120 (all in g/l, 25 ºC). Stability Decomposition
is <5% after storage for 2 years. Stable in aqueous solutions at
pH 1-9 for a test period of 35 days (50 ºC) or 14 days (80 ºC).
Slowly hydrolysed in 1N HCl and NaOH. pKa No
acid or base properties in range pH 3.5-10. F.p. No
reaction up to 360 ºC
COMMERCIALISATION
History Fungicide reported by U. Gisi et al. (Proc.
Br. Crop Prot. Conf., 1986, 1, 33; 2, 857).
Introduced in France and Switzerland (1989) by Sandoz AG (now Syngenta
AG). Patents US 4664696 Manufacturers Syngenta
APPLICATIONS
Biochemistry Steroid demethylation inhibitor. Mode of action Systemic fungicide with protective, curative, and eradicant action. Absorbed rapidly by the plant, with translocation acropetally. Uses Foliar, systemic fungicide for control of Septoria, rust, powdery mildew, Rhynchosporium, Cercospora, and Ramularia in cereals and sugar beet, at 60-100 g/ha; and rust, Mycena, Sclerotinia and Rhizoctonia in coffee and turf. Formulation types SC; SL; WG.
